ocrevus start up form
On Day 1 and Day 15 Rate. In RMS trials 58 of OCREVUS-treated patients experienced one or more infections compared to 52 of REBIF-treated patients.
Ocrevus may cause unpleasant side effects.
. Service Benefit Plan Prior Approval PO. OCREVUS is indicated for. It is a one-time registration completed by the practice.
Youll receive doses of Ocrevus from a healthcare professional by intravenous IV infusion. OCREVUS Start Form The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions. How to Take Ocrevus.
Loading doses must be administered in a controlled infusion site. Prescription Enrollment Form. Once youve written a prescription for OCREVUS complete the Start Form or enroll patients online to get them started with OCREVUS CONNECTS and begin.
Date of birth. Your healthcare provider can enroll you in this registry by calling 1-833-872-4370 or visiting. Ocrevus SGM 102020.
OCREVUS Start Form Once youve prescribed OCREVUS enroll your patients in OCREVUS Access Solutions Visit the Site The OCREVUS Co-pay Program Eligible commercially insured patients could. 2 Patient Authorization and Additional Consents KESIMPTA 0. Ad Discover The Safety Efficacy Of TYSABRI.
Ad Discover The Safety Efficacy Of TYSABRI. Use the prepared infusion solution immediately. When possible you should receive any non-live vaccines at least 2 weeks before you start treatment with OCREVUS.
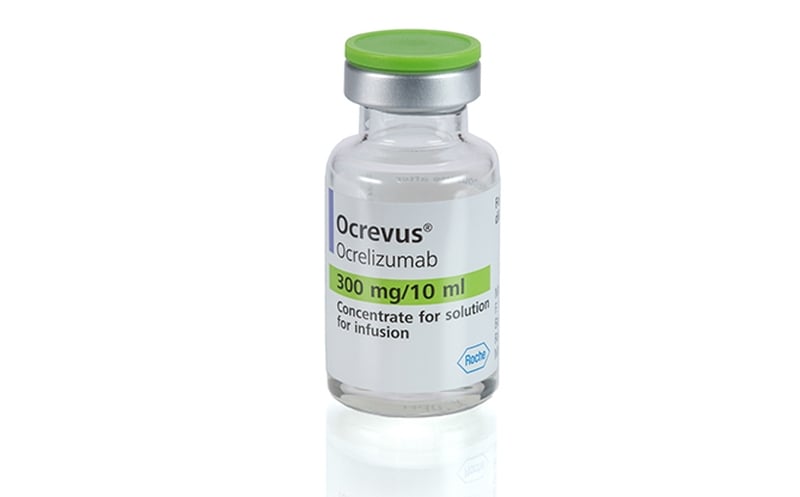
OCREVUS ocrelizumab. Prior to the start of the intravenousinfusion the content of the infusion bag shouldbe at room temperature. 300mg10mL SDV to a final concentration of 12mgmL.
OCREVUS is a prescription medicine used to treat. Ocrevus FEP CSU_MD Fax Form Revised 11152019 Send completed form to. These infusion reactions can happen for up to 24 hours after your infusion.
Start at 30mlhr increasing by 30mlhr every 30 minutes to a maximum rate of. When possible you should receive any non-live vaccines at least 2 weeks before you start treatment with OCREVUS. Ocrevus ocrelizumab Fax completed form to 8883021028.
Prescribers first name. Ad Get Reimbursement Coding Info For Your Patients Here. Ocrevus may also be used for purposes not listed in this medication guide.
Ocrevus ocrelizumab Vials are diluted in NS Initial dose two infusions Note. By completing this form you are requesting services on behalf of your patient which may include. OCREVUS Start Form Ready to get started.
Visit The Access Solutions Site. Some side effects may occur during the injection or up to. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease in.
The form includes patient insurance and prescription. It must be completed by the provider. Complete entire form and fax to Alongside KESIMPTA at 1-833-318-0680 An incomplete Start Form may delay the start of treatment.
Patients first name. As email is not a secure medium any person with concerns about their prior authorization formmedical information. For additional safety information please see the full Prescribing Information and Medication Guide.
The purpose of this registry is to collect information about your health and your babys health. Taking certain medications before receiving Ocrevus helps prevent side effects. Ad Get Reimbursement Coding Info For Your Patients Here.
See Website For Safety Boxed Warning PI. Ocrevus start up form Wednesday June 1 2022 Edit. Ocrevus ocrelizumab injection is a preservative-free sterile clear or slightly opalescent and colorless to pale brown solution supplied as a carton containing one 300 mg10 mL 30 mgmL.
In the PPMS trial 70 of OCREVUS-treated patients experienced one or more. CVS Caremark Specialty Pharmacy 2211 Sanders Road NBT-6 Northbrook IL 60062 Phone. This form is used to initiate the EFT registration process when the practice chooses not to use check reimbursements.
Box 52080 MC 139 Phoenix AZ 85072-2080 Attn. 300mg in 250ml 09 sodium chloride Frequency. If you would like to receive any non-live inactivated vaccines including the seasonal flu.
Benefits investigation Benefits reverification approximately 6 weeks prior to. See Website For Safety Boxed Warning PI. Ocrevus basics Ocrevus comes as a liquid solution inside a vial.
Ocrevus intravenous infusion Induction. Typically an antihistamine like Benadryl and corticosteroid like. This is an injection thats given.
Visit The Access Solutions Site. Ocrevus Order Form Prescriber Signature Date Please Print Name Form 350 NForms300 - PHARMACYF350 - OcrevusPhysician Order Formdocx Orders are initiated unless crossed out by.
/multiple-sclerosis-ms-disability-5200983-Final-V2-e60aa676fe194a32a2e98eb49f859a0c.jpg)
Multiple Sclerosis Ms Disability Benefits Criteria Applying
Ectrims Roche Touts 8 Year Ocrevus Data To Defend Multiple Sclerosis Leadership Fierce Pharma
Drugs In Development For Multiple Sclerosis Practical Neurology

European Multiple Sclerosis Platform We Help People Living With Ms

Ocrevus Connects Patient Support Program
![]()
Ocrevus Connects Patient Support Program

Ocrevus Ocrelizumab Ms Infusion Experience

Sign Up For Patient Support And Resources Ocrevus Ocrelizumab
Ocrevus Connects Patient Support Program

Ocrevus Side Effects Cost Uses And More

Ocrevus Ocrelizumab Ms Infusion Experience

Ocrevus Connects Patient Support Program

Chronically Ill Traumatically Billed The 123 000 Medicine For Ms Kaiser Health News
![]()
Ocrevus Connects Patient Support Program
![]()
Ocrevus Connects Patient Support Program